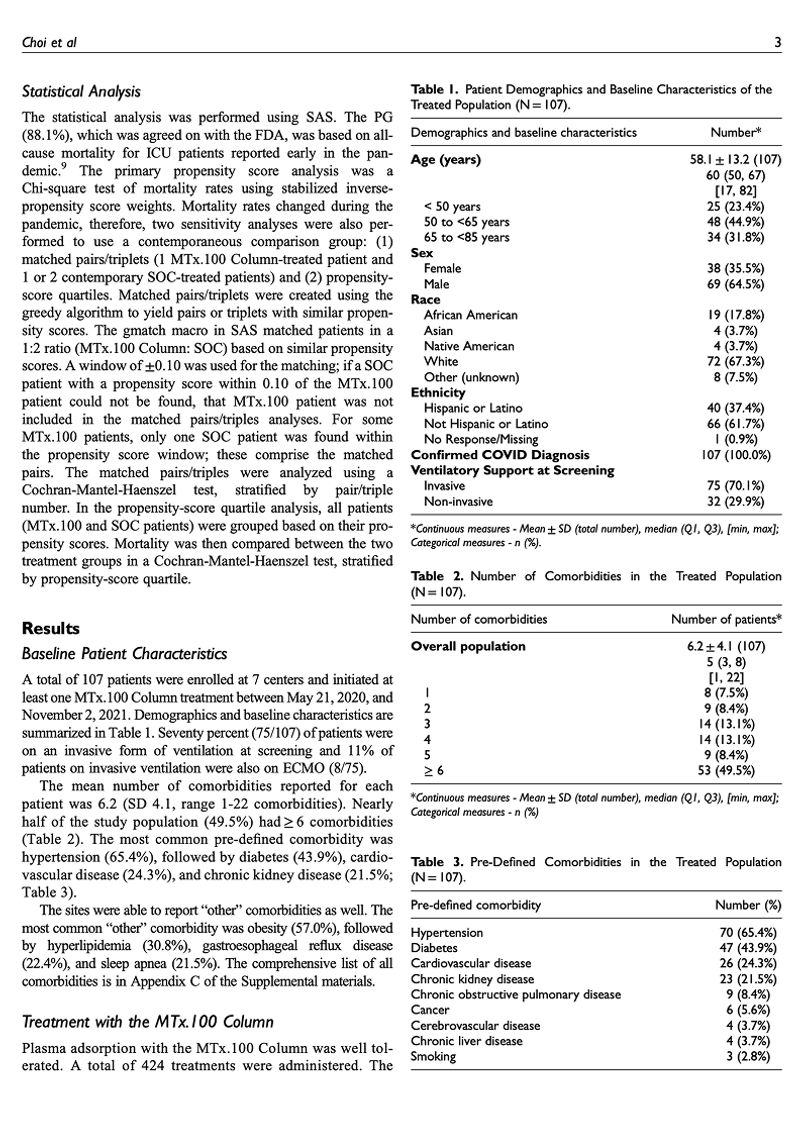
Backed By Science
Proven safe and effective
Key Clari Benefits
Gentle on Your Blood
Selective adsorption removes toxins while keeping antibodies, clotting factors, and electrolytes intact.
Unlike harsh filtration, Clari binds harmful molecules directly, avoiding hemolysis, platelet activation, and unnecessary inflammation.
Plasma-Precise
Targets only plasma for safer, more accurate treatment.
Only plasma contacts the column. Red and white cells stay untouched, reducing cellular stress.
Engineered for Safety
Backed by CE Mark indications and FDA Emergency Use Authorization.
Our proprietary non-ionic adsorbent resin preserves electrolytes and hemodynamic balance, reducing physiological stress.
CE Mark Approved
Approved Indication (CE Mark): For adjunct use in any condition that requires a reduction of inflammatory cytokines or metabolic waste products, drug overdose, and poisonings. It can also be used when acute hemodialysis, apheresis, or therapeutic plasma exchange (TPE) is prescribed by a physician.
Comparison
See how Clari stands up
| Clari |
|---|
| Clari | Cytosorb | Seraph 100 | Oxiris | INUSpheresis | EBOO | Therapeutic Plasma Exchange | Jafron HA Series | Other Devices |
|---|
| Clari | |
|---|---|
| Physical Characteristics | |
| Removal Method Extracorporeal blood purification is a therapeutic modality that involves circulating a patient's blood through an external device to remove pathogenic substances. The principles of removal fall into two main categories: size-based segregation, also known as filtration, and surface-based binding, known as adsorption. Filtration mechanisms operate like a sieve, mechanically removing particles based on their size, which can be an indiscriminate and potentially harsh process. In contrast, adsorption works by binding specific harmful molecules to a specialized material, often described as a magnet-like process. This approach is considered more targeted and gentle on blood components. 1A critical distinction exists between devices that treat whole blood and those that first separate blood into its components. Hemoperfusion is the process of circulating and purifying whole blood, allowing it to directly interact with an adsorptive material or membrane. Conversely, plasmapheresis centrifugally separates the blood into its cellular components (red and white blood cells, platelets) and plasma, the liquid fraction. In this process, only the plasma is purified before being recombined with the blood cells and returned to the body. This plasma-specific approach involves no direct between the cellular components and the adsorptive material, is considerably gentler and significantly reduces the risk of damaging blood cells or triggering an additional inflammatory response. 1 | Adsorption |
| Adsorption Materials | Selective multimedia blend, non-ionic7 |
| Surface Area (m²) More surface area means more space to grab and hold harmful substances, making it faster and more effective. Consequently, higher surface area is directly related to a device's ability to achieve more thorough and faster treatment sessions. 10 | 200,0001 |
| Procedure | |
| Exposure to Plasma Directly (vs. Whole Blood) Plasma is the liquid part of your blood, where most toxins, chemicals and their associated inflammatory molecules are carried. The benefits of blood purification must be weighed against potential risks, particularly with technologies that treat whole blood or rely on certain membrane materials. By dealing with just the plasma, the device can target the harmful substances more directly, without contacting the cellular components. This is a more gentle and effective process and reduces risk of damaging red or white blood cells, which can cause an inflammatory or toxic response. A significant concern with many extracorporeal devices is hemolysis, the destruction of red blood cells. 13Hemolysis can be an expected side effect of whole blood hemoperfusion, as these filters can damage and destroy red blood cells 14, releasing toxic inter-cellular material into the bloodstream. This is in contrast to plasma-specific treatments like the MTx.100 15, which are designed to avoid this risk. Hemolysis can also lead to secondary complications, most notably platelet activation. The breakdown of red blood cells releases ADP and cell-free hemoglobin, which can both trigger and enhance platelet activation, leading to a hypercoagulable state. 16Whole blood hemoadsorption devices, especially those relying on chemical adsorption, can cause platelet activation, as was noted in a study on CytoSorb which documented a significant decrease in platelets during therapy. 8In contrast, the treatment of only plasma with the MTx.100 15is specifically designed to avoid causing or promoting platelet activation. 16Another limitation is the issue of protein binding, or "protein fouling," on membranes and adsorbent materials. 17Some filtration membranes, such as those made of cellulose nitrate and nylon, have a high affinity for proteins, which can lead to sample loss. 18While some devices are engineered to remove pathogenic proteins, non-selective binding can also remove beneficial substances and significantly reduce the efficiency of the membrane over time. For example, some devices have been shown to remove useful molecules like albumin, fatty acids, and fat-soluble vitamins, which can lead to complications such as malnutrition. 8The MTx.100 plasma adsorption column is designed to retain important components like immunoglobulins, electrolytes, and healthy proteins, mitigating these risks. 1 | |
| Replacement Fluids Needed Donor plasma can be lifesaving in emergencies, however for an elective procedure it may not be ideal. It is a foreign (mostly human derived) substance, which could trigger an immune response and in rare cases, toxic. There is a small but real risk of allergic reactions, infections and inflammation with donor plasma as the immune system may be activated. | |
| Length of each procedure | 2 hours outpatient, 1.5 hours inpatient |
| Initial Procedures Required | 1 |
| Claimed Key Substances Removed | Microplastics, PFAS, immune toxins, metabolic waste, broad spectrum environmental toxins, cytokines, inflammatory mediators, pesticides, poisons, drug toxins1, 19 |
| Common Setting | ICU, hospitals or private clinic |
| Clinical Evidence | |
| Regulatory Status (EU) | CE Mark (broad)7 |
| Regulatory Status (US / Canada) | FDA EUA (COVID-19)19 |
| Clinical Evidence Level The clinical evidence supporting the MTx.100 plasma adsorption column’s efficacy is notable. A prospective, single-arm, multicenter EUA trial in 107 critically ill COVID-19 patients in the ICU demonstrated a 28-day all-cause mortality of 37.4%, a significant improvement over the prespecified performance goal of 88.1%. 1A propensity score-matched (PSM) analysis further showed that survival odds were three times higher for patients treated with the MTx.100 plasma adsorption column compared to a contemporaneous cohort receiving standard of care alone. 1The trial also reported no serious adverse events attributable to the device or procedure, highlighting a strong safety profile. 1This level of prospective, comparative clinical data is a significant advantage in a field where many devices are supported by smaller case series or in-vitro studies. This robust evidence, though from an acute care setting, provides a strong foundation for its potential application in the emerging chronic and wellness market, where its safety profile and broad-spectrum capabilities are highly relevant. | Published EUA trial (n=107) w/ PSM analysis, case series early clinical trials, including FDA IDE trials37 |
| Pros | Private clinic and hospital use. Highest surface area; broad-spectrum removal; rapid, gentle procedure; no donor plasma needed; strong clinical data on safety and mortality |
| Cons | No removal of circulating immunoglobin antibodies1 |
| Clari | Cytosorb | Seraph 100 | Oxiris | INUSpheresis | EBOO | Therapeutic Plasma Exchange | Jafron HA Series | Other Devices | |
|---|---|---|---|---|---|---|---|---|---|
| Physical Characteristics | |||||||||
| Removal Method Extracorporeal blood purification is a therapeutic modality that involves circulating a patient's blood through an external device to remove pathogenic substances. The principles of removal fall into two main categories: size-based segregation, also known as filtration, and surface-based binding, known as adsorption. Filtration mechanisms operate like a sieve, mechanically removing particles based on their size, which can be an indiscriminate and potentially harsh process. In contrast, adsorption works by binding specific harmful molecules to a specialized material, often described as a magnet-like process. This approach is considered more targeted and gentle on blood components. 1A critical distinction exists between devices that treat whole blood and those that first separate blood into its components. Hemoperfusion is the process of circulating and purifying whole blood, allowing it to directly interact with an adsorptive material or membrane. Conversely, plasmapheresis centrifugally separates the blood into its cellular components (red and white blood cells, platelets) and plasma, the liquid fraction. In this process, only the plasma is purified before being recombined with the blood cells and returned to the body. This plasma-specific approach involves no direct between the cellular components and the adsorptive material, is considerably gentler and significantly reduces the risk of damaging blood cells or triggering an additional inflammatory response. 1 | Adsorption | Adsorption | Surface heparin receptor binding2 | Filtration and non-selective membrane Adsorption3 | Double membrane Filtration4 | Ozonation & Filtration | Plasma Exchange | Adsorption5 | Adsorption/Filtration6 |
| Adsorption Materials | Selective multimedia blend, non-ionic7 | Porous polystyrene-divinylbenzene polymer beads, ionic8 | Heparin-coated beads2 | AN69 membrane, PEI layer3 | Double membrane filtration4 | N/A | No filter or adsorption | Double cross-linked styrene-divinylbenzene copolymers5 | Varies (e.g., Kaneka LDL removal)9 |
| Surface Area (m²) More surface area means more space to grab and hold harmful substances, making it faster and more effective. Consequently, higher surface area is directly related to a device's ability to achieve more thorough and faster treatment sessions. 10 | 200,0001 | 40,00011 | 0.8* | 150010 | Estimated 1-4* | Estimated 0.7-1.0* | N/A | N/A (adsorbent volume listed)5 | Varies12 |
| Procedure | |||||||||
| Exposure to Plasma Directly (vs. Whole Blood) Plasma is the liquid part of your blood, where most toxins, chemicals and their associated inflammatory molecules are carried. The benefits of blood purification must be weighed against potential risks, particularly with technologies that treat whole blood or rely on certain membrane materials. By dealing with just the plasma, the device can target the harmful substances more directly, without contacting the cellular components. This is a more gentle and effective process and reduces risk of damaging red or white blood cells, which can cause an inflammatory or toxic response. A significant concern with many extracorporeal devices is hemolysis, the destruction of red blood cells. 13Hemolysis can be an expected side effect of whole blood hemoperfusion, as these filters can damage and destroy red blood cells 14, releasing toxic inter-cellular material into the bloodstream. This is in contrast to plasma-specific treatments like the MTx.100 15, which are designed to avoid this risk. Hemolysis can also lead to secondary complications, most notably platelet activation. The breakdown of red blood cells releases ADP and cell-free hemoglobin, which can both trigger and enhance platelet activation, leading to a hypercoagulable state. 16Whole blood hemoadsorption devices, especially those relying on chemical adsorption, can cause platelet activation, as was noted in a study on CytoSorb which documented a significant decrease in platelets during therapy. 8In contrast, the treatment of only plasma with the MTx.100 15is specifically designed to avoid causing or promoting platelet activation. 16Another limitation is the issue of protein binding, or "protein fouling," on membranes and adsorbent materials. 17Some filtration membranes, such as those made of cellulose nitrate and nylon, have a high affinity for proteins, which can lead to sample loss. 18While some devices are engineered to remove pathogenic proteins, non-selective binding can also remove beneficial substances and significantly reduce the efficiency of the membrane over time. For example, some devices have been shown to remove useful molecules like albumin, fatty acids, and fat-soluble vitamins, which can lead to complications such as malnutrition. 8The MTx.100 plasma adsorption column is designed to retain important components like immunoglobulins, electrolytes, and healthy proteins, mitigating these risks. 1 | Varies (Majority Whole Blood) | ||||||||
| Replacement Fluids Needed Donor plasma can be lifesaving in emergencies, however for an elective procedure it may not be ideal. It is a foreign (mostly human derived) substance, which could trigger an immune response and in rare cases, toxic. There is a small but real risk of allergic reactions, infections and inflammation with donor plasma as the immune system may be activated. | |||||||||
| Length of each procedure | 2 hours outpatient, 1.5 hours inpatient | Continuous, for up to 12 hours per cartridge | Continuous, for up to 24 hours per cartridge | Continuous, 24-96 hours | 3-4 hours | 1-1.5 hours | 2-4 hours | 2-5 hours | Varies |
| Initial Procedures Required | 1 | 2-4+ | 1-4+ | 1-4+ | 2+ | 2-14+ | 6-9+ | 2-6+ | Varies |
| Claimed Key Substances Removed | Microplastics, PFAS, immune toxins, metabolic waste, broad spectrum environmental toxins, cytokines, inflammatory mediators, pesticides, poisons, drug toxins1, 19 | Cytokines, myoglobin, endotoxins8, 20 | Bacteria, viruses, fungi, pathogens2 | Endotoxins, cytokines3 | Autoantibodies, inflammatory cytokines, oxidized LDLs, environmental toxins, pathogenic substances, general toxins, and large molecules.4, 21 | Bacteria, viruses (via ozone)22 | Autoantibodies, immune complexes,23electrolytes, albumin, clotting factors 24 | Drugs, poisons, biotoxins, chemotherapy cytostatics, pesticides, rodenticides, acute poisoning5cytokines 25 | Specific proteins, LDL-C, β₂-microglobulin26 |
| Common Setting | ICU, hospitals or private clinic | ICU | ICU | ICU | Private Clinics | Private clinics | Hospital or private clinics | ICU | Hospital |
| Clinical Evidence | |||||||||
| Regulatory Status (EU) | CE Mark (broad)7 | CE Mark27 | CE Mark28 | CE Mark29 | Not verified/not publicly confirmed4 | Unregulated30 | CE Mark | CE Mark certification31 | Varies |
| Regulatory Status (US / Canada) | FDA EUA (COVID-19)19 | FDA EUA (COVID-19)27 | FDA EUA (COVID-19)2 | FDA EUA (COVID-19)10 | Not Approved32 | FDA-prohibited33 | FDA Market Clearance - In the US, TPE itself is not directly FDA-approved; instead it is the apheresis devices used to perform TPE that have FDA Market Clearance34 | Canada: Health Canada authorization35 | Varies (e.g. Kaneka FDA-approved26, Aethlon investigational) 36 |
| Clinical Evidence Level The clinical evidence supporting the MTx.100 plasma adsorption column’s efficacy is notable. A prospective, single-arm, multicenter EUA trial in 107 critically ill COVID-19 patients in the ICU demonstrated a 28-day all-cause mortality of 37.4%, a significant improvement over the prespecified performance goal of 88.1%. 1A propensity score-matched (PSM) analysis further showed that survival odds were three times higher for patients treated with the MTx.100 plasma adsorption column compared to a contemporaneous cohort receiving standard of care alone. 1The trial also reported no serious adverse events attributable to the device or procedure, highlighting a strong safety profile. 1This level of prospective, comparative clinical data is a significant advantage in a field where many devices are supported by smaller case series or in-vitro studies. This robust evidence, though from an acute care setting, provides a strong foundation for its potential application in the emerging chronic and wellness market, where its safety profile and broad-spectrum capabilities are highly relevant. | Published EUA trial (n=107) w/ PSM analysis, case series early clinical trials, including FDA IDE trials37 | Meta-analysis (n=1297), case series, registries8 | Case series, registries, first-in-human studies2 | Case series (n=3), in-vitro studies3 | Expert testimonials, small internal studies, high treatment volume claims38 | Small case series, animal studies, no large-scale RCTs22 | Systematic reviews, meta-analyses, RCTs for specific indications23 | Case studies (e.g., MTX overdose)30in-vitro studies 25pilot studies 39 | Varies widely by device |
| Pros | Private clinic and hospital use. Highest surface area; broad-spectrum removal; rapid, gentle procedure; no donor plasma needed; strong clinical data on safety and mortality | ICU and Cardiac surgery use, removes range of inflammatory mediators8 | Direct pathogen removal ("source control"); broad-spectrum binding2 | Combines renal support with cytokine/endotoxin removal3 | Removes wide range of chronic toxins; no donor plasma needed40 | Inexpensive; simple procedure; claims of anti-inflammatory effects41 | Effective for specific autoimmune diseases; established efficacy34 | Specialized cartridges for specific pathologies; growing international approvals5 | Highly targeted for niche indications with established efficacy26 |
| Cons | No removal of circulating immunoglobin antibodies1 | Contested mortality data in meta-analysis; removes platelets/albumin8 | Trial reported significant adverse events10Primarily for pathogens; limited evidence for general inflammation 2 | Whole blood procedure3; small surface area; ICU-only use, no pathogen clearance 10 | Lack of large-scale, peer-reviewed clinical trials (n=27)21; anecdotal evidence 4 | FDA-prohibited; significant safety concerns and lack of evidence33 | Requires donor plasma/albumin; removes clotting factors, electrolytes, and antibodies24 | Less efficient for cytokine removal than competitors in one study25 | Narrow indications; not applicable for general detoxification9 |
*Derived from disclosed data, not directly reported in source publications.
Plasma Adsorption with the MTx.100 Column in Critically Ill COVID-19 Patients, accessed on August 25, 2025, https://scholarcommons.towerhealth.org/dept_pulmcrit_read/2/
Seraph 100 Microbind Affinity Blood Filter for Persistent Pediatric BK Virus Nephropathy, accessed on August 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11871063/
Hemoperfusion Using the Oxiris Membrane in Septic Shock Patients with Preserved Kidney Function: A Case Series - PubMed Central, accessed on August 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11942976/
Blood Plasma Filter Technology | Inuspheresis®, accessed on August 25, 2025, https://www.inuspheresis.com/technology
HA230 - ICU Works, accessed on August 25, 2025, https://www.icuworks.com/product/ha230/
Beta2-microglobulin-selective adsorbent column (Lixelle) for the treatment of dialysis-related amyloidosis - PubMed, accessed on August 25, 2025, https://pubmed.ncbi.nlm.nih.gov/12921124/
MTx.100 Plasma Adsorption Column - Marker Health, accessed on August 25, 2025, https://markerhealth.com/product/inflammatory-reduction-device/
CytoSorb® Hemadsorption in Cardiogenic Shock: A Real-World Analysis of Hemodynamics, Organ Function, and Clinical Outcomes During Mechanical Circulatory Support - MDPI, accessed on August 25, 2025, https://www.mdpi.com/2227-9059/13/2/324
Blood purification | Health Care Solutions Unit | Business | KANEKA CORPORATION, accessed on August 25, 2025, https://www.kaneka.co.jp/en/business/healthcare/med_002.html
A Review of Extracorporeal Blood Purification Techniques for the Treatment of Critically Ill Coronavirus Disease 2019 Patients - PMC - PubMed Central, accessed on August 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9521577/
Clinical improvement of Long-COVID is associated with reduction in autoantibodies, lipids and inflammation following therapeutic apheresis, accessed on September 16th, 2025, https://www.nature.com/articles/s41380-023-02084-1#citeas
Disposables and fluids - Fresenius Medical Care, accessed on August 25, 2025, https://freseniusmedicalcare.com/en/healthcare-professionals/critical-care/acute-blood-purification/acute-disposables-fluids/
Hemolysis: Types, Causes & Symptoms - Cleveland Clinic, accessed on August 25, 2025, https://my.clevelandclinic.org/health/diseases/24108-hemolysis
Haemoperfusion: mechanisms, indications, and complications - Deranged Physiology, accessed on August 25, 2025, https://derangedphysiology.com/main/required-reading/renal-intensive-care/Chapter-314/haemoperfusion-mechanisms-indications-and-complications
MTx.100 Plasma Adsorption Column Instructions For Use | Marker Health, accessed on August 25, 2025, https://www.markerhealth.com/wp-content/uploads/2019/09/l.039-g-instructions-for-use-english-mtx.100-plasma-adsorption-column.pdf
Mechanisms of Hemolysis-Associated Platelet Activation - UR Scholarship Repository, accessed on August 25, 2025, https://scholarship.richmond.edu/physics-faculty-publications/94/
Ultrafiltration Membrane Selection Guide for Protein Concentration - Patsnap Synapse, accessed on August 25, 2025, https://synapse.patsnap.com/article/ultrafiltration-membrane-selection-guide-for-protein-concentration
Filter Membranes - Sigma-Aldrich, accessed on August 25, 2025, https://www.sigmaaldrich.com/US/en/products/filtration/laboratory-filter-membranes/filter-membranes
Marker Health, accessed on August 25, 2025, https://www.markerhealth.com/
Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb sorbent porous polymer beads - ResearchGate, accessed on August 25, 2025, https://www.researchgate.net/publication/322710729_Broad_adsorption_of_sepsis-related_PAMP_and_DAMP_molecules_mycotoxins_and_cytokines_from_whole_blood_using_CytoSorb_sorbent_porous_polymer_beads
Clinical improvement of Long-COVID is associated with reduction in autoantibodies, lipids and inflammation following therapeutic apheresis, accessed on September 16th, 2025, https://www.nature.com/articles/s41380-023-02084-1#citeas
Extracorporeal Blood Oxygenation and Ozonation (EBOO) in Man. Preliminary Report | Request PDF - ResearchGate, accessed on August 25, 2025, https://www.researchgate.net/publication/12574128_Extracorporeal_Blood_Oxygenation_and_Ozonation_EBOO_in_Man_Preliminary_Report
Influence of therapeutic plasma exchange treatment on short-term mortality of critically ill adult patients with sepsis-induced organ dysfunction: a systematic review and meta-analysis - PubMed, accessed on August 25, 2025, https://pubmed.ncbi.nlm.nih.gov/38178170/
Pilot Study on the Replacement of Fibrinogen with Fibrinogen Concentrates During Therapeutic Plasma Exchange with Mild to Moderate Bleeding Risk—A Comparison with Fresh Frozen Plasma and Albumin Replacement, accessed on September 17, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11676064/
Adsorptive Therapies in Sepsis and Inflammation: Description of the Various Adsorptive Techniques and their Failure to Improve Outcomes - SciELO México, accessed on August 25, 2025, https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0034-83762023000600359
LIPOSORBER® | Treatment For Cardiovascular & Kidney Disease, accessed on August 25, 2025, https://liposorber.com/
CytoSorb ® 300 mL Device Authorized by FDA for Emergency Treatment of COVID-19, accessed on August 25, 2025, https://www.fda.gov/media/136866/download
Seraph® 100 Microbind® Affinity Blood Filter - Cardiolink, accessed on August 25, 2025, https://www.cardiolinkgroup.com/wp-content/uploads/2020/04/ExThera-Medical-Brochure.pdf
Baxter oXiris Label Expansion, accessed on September 17, 2025, https://www.baxteritalia.it/sites/g/files/ebysai1416/files/2017-11/09-26-17-oXiris-label-expansion.pdf
Case Report: Effective methotrexate removal by combined hemodialysis and polymeric resin hemoadsorption - Frontiers, accessed on August 25, 2025, https://www.frontiersin.org/journals/nephrology/articles/10.3389/fneph.2025.1644079/full
Good News!Jafron receives MDR certificate!_News Center_Media & Events_Health Technology For A Better Life, accessed on August 25, 2025, https://www.jafroninternational.com/events__media/news_center/837.html
Inuspheresis - Apheresis Center, accessed on August 25, 2025, https://apheresiscenter.eu/inuspheresis
Neurological Crisis Following Intravenous Ozone Therapy: A Case Report, accessed on September 17, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11868665/
(PDF) Therapeutic plasma exchange for the treatment of systemic sclerosis: A comprehensive review and analysis - ResearchGate, accessed on August 25, 2025, https://www.researchgate.net/publication/323671284_Therapeutic_plasma_exchange_for_the_treatment_of_systemic_sclerosis_A_comprehensive_review_and_analysis
Jafron Biomedical Receives Health Canada Authorization of its Disposable Hemoperfusion Cartridges for Uses Related to COVID-19_News Center_Media & Events_Health Technology For A Better Life, accessed on August 25, 2025, https://www.jafroninternational.com/events__media/news_center/74.html
The Hemopurifier® in Infectious Disease - Aethlon Medical, Inc., accessed on August 25, 2025, https://www.aethlonmedical.com/the-hemopurifier/the-hemopurifier-in-infectious-disease
Plasma Adsorption with the MTx.100 Column in Critically Ill COVID-19 Patients: A Prospective Study and Propensity Score Analysis | Request PDF - ResearchGate, accessed on August 25, 2025, https://www.researchgate.net/publication/384014042_Plasma_Adsorption_with_the_MTx100_Column_in_Critically_Ill_COVID-19_Patients_A_Prospective_Study_and_Propensity_Score_Analysis
Applications of Inuspheresis® | Plasma Purification, accessed on August 25, 2025, https://www.inuspheresis.com/applications?d7df0e6b_page=3
Comparison of the CytoSorb® 300 mL and Jafron HA380 hemoadsorption devices: an in vitro study | Request PDF - ResearchGate, accessed on August 25, 2025, https://www.researchgate.net/publication/362421585_Comparison_of_the_CytoSorbR_300_mL_and_Jafron_HA380_hemoadsorption_devices_an_in_vitro_study
Inuspheresis® | Plasma Purification, accessed on August 25, 2025, https://www.inuspheresis.com/procedure
(PDF) Extracorporeal blood oxygenation and ozonation: Clinical and ..., accessed on August 25, 2025, https://www.researchgate.net/publication/7605837_Extracorporeal_blood_oxygenation_and_ozonation_Clinical_and_biological_implications_of_ozone_therapy
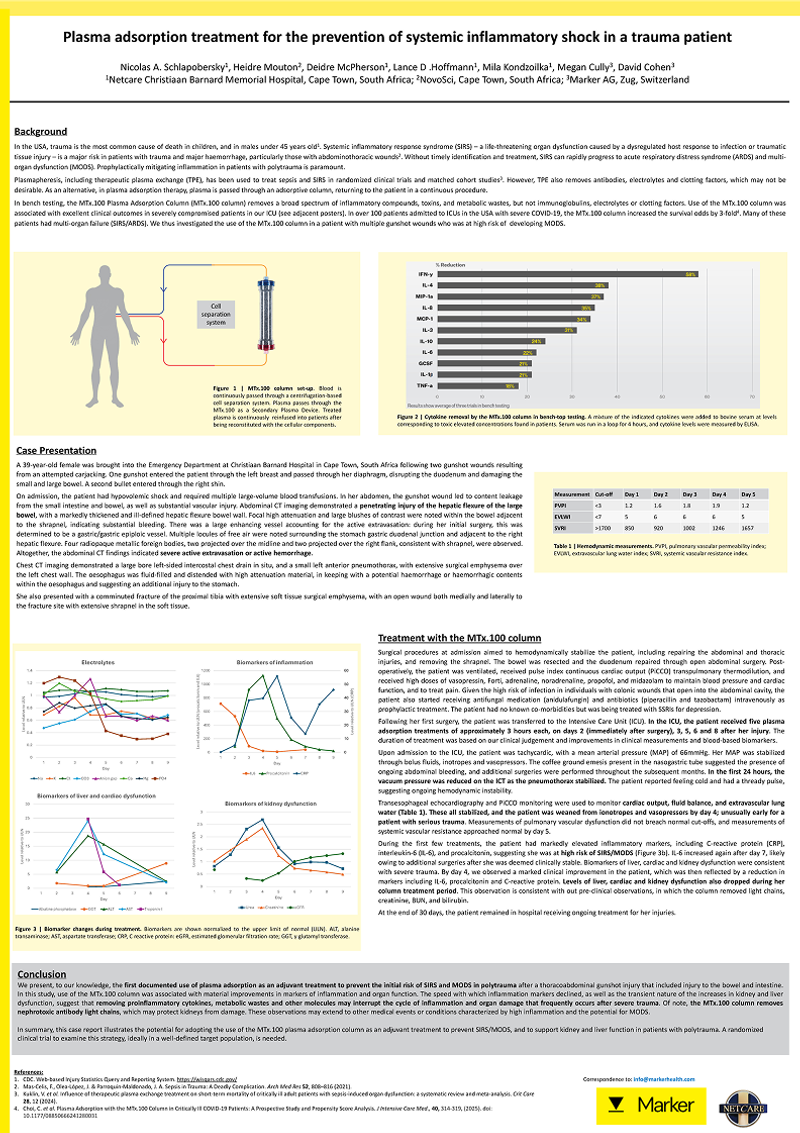
Plasma Adsorption for Critical COVID-19 Patients
Early in the COVID-19 pandemic, patients with severe disease admitted to the intensive care unit (ICU) had a high incidence of mortality. We aimed to investigate whether plasma adsorption with the MTx.100 Column could improve survival.
We demonstrated threefold increase in the odds of survival versus standard of care.
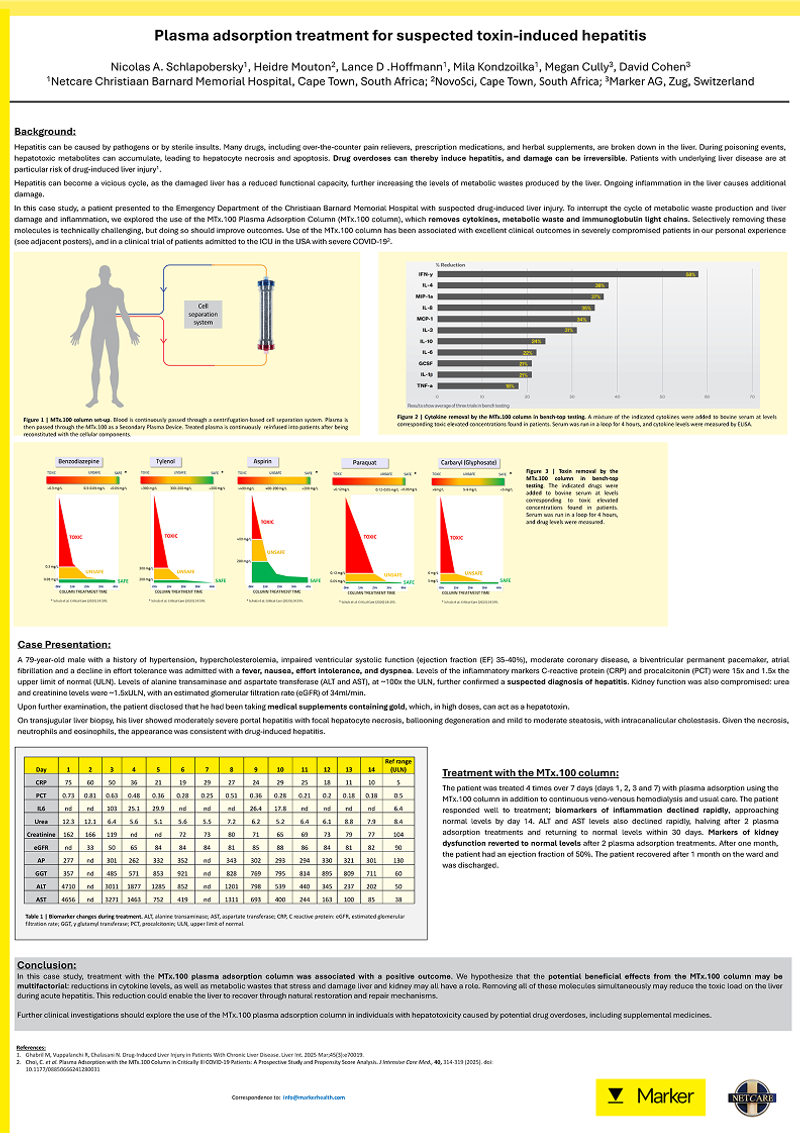
Research & Clinical Case Studies
Explore our published case studies demonstrating the use of the MTx.100 plasma adsorption column in different clinical settings. These peer-reviewed posters highlight how the therapy can reduce inflammation, improve patient outcomes, and support recovery across toxin-induced hepatitis, trauma-related systemic inflammation, and LVAD implantation.
Microplastics
Microplastics enter our bodies through food, water, and the air we breathe. Once inside, they aren’t easily broken down, and can trigger inflammation, disrupt hormones, and may carry toxic chemicals that increase the risk of cancer, organ damage, and immune system issues. Over time, the accumulation of microplastics may pose serious, long-term health risks.
Zhang, C. et al. Association of mixed exposure to microplastics with sperm dysfunction: a multi-site study in China. EBioMedicine 108, 105369 (2024).
Marfella, R. et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. New England Journal of Medicine 390, 900–910 (2024).
Kwon, J.-H. et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int J Environ Res Public Health 17, 6710 (2020).
Wright, S. L., Ulke, J., Font, A., Chan, K. L. A. & Kelly, F. J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int 136, 105411 (2020).
Periyasamy, A. P. & Tehrani-Bagha, A. A review on microplastic emission from textile materials and its reduction techniques. Polym Degrad Stab 199, 109901 (2022).
Campen, M. et al. Bioaccumulation of Microplastics in Decedent Human Brains Assessed by Pyrolysis Gas Chromatography-Mass Spectrometry. Res Sq (2024) doi:10.21203/ rs.3.rs-4345687/v1
Hu, C. J. et al. Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis. Toxicol Sci 200, 235–240 (2024).
Winiarska, E., Jutel, M. & Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ Res 251, 118535 (2024).
Inam, Ö. et al. Impact of microplastics on female reproductive health: insights from animal and human experimental studies: a systematic review. Arch. Gynecol. Obstet. 312, 77–92 (2025).
Hong, Y. et al. Adverse effects of microplastics and nanoplastics on the reproductive system: A comprehensive review of fertility and potential harmful interactions. Sci. Total Environ.903, 166258 (2023).
Hunt, K. et al. Exposure to microplastics and human reproductive outcomes: a systematic review. BJOG131, 675–683 (2024).
Balali, H., Morabbi, A. & Karimian, M. Concerning influences of micro/nano plastics on female reproductive health: focusing on cellular and molecular pathways from animal models to human studies. Reprod. Biol. Endocrinol.22, 141 (2024).
Ali, W. et al. A critical review on male‑female reproductive and developmental toxicity induced by micro‑plastics and nano‑plastics through different signaling pathways. Environmental Pollution 333, 122024 (2024).
Nihart, A. J. et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med.31, 1114–1119 (2025).
Pelepenko, L. et al. Effects of microplastics on the bones: a comprehensive review. Osteoporos. Int.36, 1327–1345 (2025).
Lee, Y.‑H., Zheng, C.‑M., Wang, Y.‑J. et al. Effects of microplastics and nanoplastics on the kidney and cardiovascular system. Nat. Rev. Nephrol.21, 585–596 (2025).
Hampson, H. E. et al. The potential mediating role of the gut microbiome and metabolites in the association between PFAS exposure and kidney function in young adults: a proof‑of‑concept study. Sci. Total Environ.954, 176519 (2024).
Liu, H. et al. Micro/nano-plastics cause neurobehavioral toxicity in discus fish via the brain-gut-microbiota axis. Aquat. Toxicol. 421, 126830 (2022).
Marfella, R. et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 390, 900–910 (2024).
Thompson, R. C. et al. Twenty years of microplastic pollution research — what have we learned Science 386, eadl2746 (2024).
Ali, N., Katsouli, J., Marczylo, E. L., Gant, T. W., Wright, S. & Bernardino de la Serna, J. The potential impacts of micro‑ and nano‑plastics on various organ systems in humans. EBioMedicine 99, 104901 (2024).
Yan, Z. et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol.56, 414–421 (2022).
Hernandez, L. M. et al. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol.53, 12300–12310 (2019).
Milne, M. H. et al. Exposure of U.S. adults to microplastics from commonly‑consumed proteins. Environ. Pollut. 343, 123233 (2024).
Habumugisha, T. et al. Uptake, bioaccumulation, biodistribution and depuration of polystyrene nanoplastics in zebrafish (Danio rerio). Sci. Total Environ.893, 164840 (2023).
Cox, K. D. et al. Human consumption of microplastics. Environ. Sci. Technol.53, 7068–7074 (2019).
Kacprzak, S. & Tijing, L. D. Microplastics in the indoor environment: sources, mitigation and fate. J. Environ. Chem. Eng.10, 107359 (2022).
Hussain, K. A. et al. Assessing the release of microplastics and nanoplastics from plastic containers and reusable food pouches: implications for human health. Environ. Sci. Technol.57, 9782–9792 (2023).
Forever Chemicals
Forever chemicals, or PFAS (per- and polyfluoroalkyl substances) are harmful because they don’t break down quickly in the environment, or in our bodies. Accumulation over time may pose serious long-term health risks and lead to cancer, immune system issues, and hormone disruption.
D’Ambro, E. L., Murphy, B. N., Bash, J. O., Gilliam, R. C. & Pye, H. O. T. Predictions of PFAS regional-scale atmospheric deposition and ambient air exposure. Science of The Total Environment 902, 166256 (2023).
Piva, E. et al. Per- and polyfluoroalkyl substances (PFAS) presence in food: Comparison among fresh, frozen and ready-to-eat vegetables. Food Chem 410, 135415 (2023).
Ragnarsdóttir, O., Abdallah, M. A.-E. & Harrad, S. Dermal uptake: An important pathway of human exposure to perfluoroalkyl substances? Environmental Pollution 307, 119478 (2022).
Fenton, S. E. et al. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 40, 606–630 (2021).
European Environment Agency. Risks of PFAS for human health in Europe (Signal). https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/risk-of-pfas-in-humans (2024).
Gaillard, L., Barouki, R., Blanc, E., Coumoul, X. & Andréau, K. Per- and polyfluoroalkyl substances as persistent pollutants with metabolic and endocrine-disrupting impacts. Trends Endocrinol Metab (2024) doi:10.1016/j.tem.2024.07.021
Lockington, C. & Favetta, L. A. How Per- and Poly-Fluoroalkyl Substances Affect Gamete Viability and Fertilization Capability: Insights from the Literature. J Xenobiot 14, 651–678 (2024).
Baker, N. A. et al. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect 121, 105–10 (2013).
Pascali J et al. Per- and polyfluoroalkyl substances (PFAS) presence in food: Comparison among fresh, frozen and ready-to-eat vegetables - PubMed Food Chem 410, (2023)
Qu, R. et al.Per- and Polyfluoroalkyl Substances (PFAS) Affect Female Reproductive Health: Epidemiological Evidence and Underlying Mechanisms.Toxics 12, 678 (2024)
Wang, W., Hong, X., Zhao, F. & Wu, J. The effects of perfluoroalkyl and polyfluoroalkyl substances on female fertility: a systematic review and meta-analysis. Environ. Res.216, 114718 (2023).
Cohen, N.J. et al. Exposure to perfluoroalkyl substances and women’s fertility outcomes in a Singaporean population‑based preconception cohort. Sci. Total Environ. 873, 162267 (2023).
Sun, Z., Wen, Y., Wang, B., Deng, S., Zhang, F., Fu, Z., Yuan, Y. & Zhang, D. Toxic effects of per‑ and polyfluoroalkyl substances on sperm: Epidemiological and experimental evidence. Front. Endocrinol.14, 1114463 (2023).
González‑Alvarez, M. E., Antwi‑Boasiako, C. & Keating, A. F. Effects of Per‑ and Polyfluoroalkylated Substances on Female Reproduction.Toxics 12, 455 (2024).
Luo, R. et al. Associations of exposure to bisphenol‑A or parabens with markers of liver injury/function among US adults in NHANES 2011‑2016.J. Expo. Sci. Environ. Epidemiol. 35, 611–618 (2025).
Guillette, T. C.et al. Blood concentrations of per‑ and polyfluoroalkyl substances are associated with autoimmune‑like effects in American alligators from Wilmington, North Carolina. Front. Toxicol.10, 1010185 (2022).
Ehrlich, V., Bil, W., Vandebriel, R. et al. Consideration of pathways for immunotoxicity of per‑ and polyfluoroalkyl substances (PFAS).Environ. Health 22, 19 (2023).
He, Y., Qu, C., Tian, J. et al. Association of perfluoroalkyl and polyfluoroalkyl substances (PFASs) exposures and the risk of systemic lupus erythematosus: a case–control study in China. Environ. Health22, 78 (2023).
Kosarek, N. N. & Preston, E. V. Contributions of synthetic chemicals to autoimmune disease development and occurrence. Curr. Environ. Health Rep.11, 128–144 (2024).
Huang, R.-G. et al. Endocrine‑disrupting chemicals and autoimmune diseases. Environ. Res.231, 116222 (2023).
Hong, Y. et al. Environmental triggers and future risk of developing autoimmune diseases: molecular mechanism and network toxicology analysis of bisphenol A. Sci. Total Environ.882, 164960 (2025).
Cimmino, I., Fiory, F., Perruolo, G., Miele, C., Beguinot, F., Formisano, P. & Oriente, F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci.21, 5761 (2020).
Nabi, M. & Tabassum, N. Role of environmental toxicants on neurodegenerative disorders. Front. Toxicol.4, 837579 (2022).
Aderinto, N., Ajagbe, A. O., Olatunji, G. D., Ogieuhi, I. J. et al. The impact of air pollution on neurodegenerative diseases: a narrative review of current evidence. Egypt J. Intern. Med.37, 18 (2025).
Jansen, A. et al. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss — a review. J. Toxicol. Environ. Health B Crit. Rev. 20, 22–37 (2017).
Fénichel, P. et al. Sustained bloodstream release of persistent organic pollutants induced by extensive weight loss after bariatric surgery: implications for women of childbearing age. Environ. Int.151, 106400 (2021).
Carwile, J. L. et al. Canned soup consumption and urinary Bisphenol A: a randomized crossover trial. JAMA306, 2218–2220 (2011).
Genuis, S. J., Beesoon, S., Birkholz, D. & Lobo, R. A. Human excretion of bisphenol A: blood, urine, and sweat (BUS) study. J. Environ. Public Health2012, 185731 (2012).
Pesticides & Herbicides
Herbicides and pesticides contain chemicals that can harm human health, especially with long-term exposure. These substances are linked to respiratory issues, hormone disruption, and an increased risk of cancers and neurological disorders. Regular exposure, even in small amounts can accumulate in the body, leading to chronic health problems.
Vasseur, C. et al. Glyphosate presence in human sperm: First report and positive correlation with oxidative stress in an infertile French population. Ecotoxicol Environ Saf 278, 116410 (2024).
Kurrasch et al. Glyphosate toxicity: in vivo, in vitro, and epidemiological evidence, Toxicological Sciences, Volume 192, Issue 2, Pages 131–140 (2023)
Marcoccia et al. Overview of human health effects related to glyphosate exposure. Front. Toxicol. Volume 6 (2024)
Lushchak O. et al. The effects of low-toxic herbicide Roundup and glyphosate on mitochondria - PMC. EXCLI Journal Jan Pages 183-196 (2022) 2022 Jan 10;21:183–196